Since the advent of the touch era marked by Apple products, capacitive touchscreen solutions have become the mainstream for human-computer interaction in mobile terminals such as smartphones, tablets, and touchscreen laptops, thanks to their stable performance and good touch feel. Regardless of the touch technology used, the cover plate is an essential protective component. Cover Glass (cover plate glass) has gradually become the mainstream choice for cover plates due to its high light transmittance, strong scratch resistance, and other properties.
The core differences between cover glass substrates stem from their production formulas. The mainstream types are soda-lime glass and high-alumina silicate glass (referred to as "high-alumina glass" for short). Both, along with the commonly used "borosilicate glass," belong to the silicate glass family. However, subtle adjustments in their chemical compositions result in vastly different physical properties and application scenarios—a typical example of "composition determining performance."
Soda-lime glass is a common type of glass, accounting for approximately 90% of global glass production. It is widely used in everyday items such as window glass, beverage bottles, and ordinary home appliance panels. Its composition uses silicon dioxide (SiO₂) as the "framework" (content: 70%-75%), which is the basis for the glass’s transparency and hardness. Additionally, it contains sodium oxide (Na₂O, 12%-15%) and calcium oxide (CaO, 8%-10):
- Sodium oxide acts as a "fluxing agent," reducing the melting temperature of glass from 1723°C (for pure silicon dioxide) to approximately 1500°C, significantly lowering energy consumption.
- Calcium oxide serves as a "stabilizer," enhancing the glass’s chemical stability and preventing it from dissolving in water.
However, the alumina content in soda-lime glass is usually less than 13%, leading to low atomic arrangement density and inherently weak impact resistance and chemical corrosion resistance. Nevertheless, its raw materials (quartz sand, soda ash, limestone) are easily available, and its production does not require complex composition control. With a cost only 1/3 to 1/2 of that of high-alumina glass, it becomes the first choice for cost-sensitive scenarios.
While retaining the silicon dioxide framework, high-alumina glass increases its alumina (Al₂O₃) content to 12%-13%. This key adjustment brings about an overall leap in performance:
- Alumina optimizes atomic arrangement density and enhances intermolecular bonding force, significantly improving the glass’s mechanical strength. It also reduces the coefficient of thermal expansion (usually ≤85×10⁻⁷/°C, much lower than that of soda-lime glass), preventing the glass from cracking under sudden temperature changes (e.g., moving from an outdoor environment of -40°C to a warm indoor space).
- Its light transmittance can reach 91.5%-92%, with extremely low impurity content and near-transparent color, enabling accurate reproduction of screen colors—this is the core reason why it is preferred for high-end smartphones and in-vehicle screens.
However, high performance comes at a cost: the high alumina content raises the melting temperature to over 1600°C, and precise control of composition uniformity is required (otherwise, defects such as bubbles and stones are likely to occur). This significantly increases the complexity and cost of the production process compared to soda-lime glass.

If composition is the "gene," physical properties are the "external manifestation." The gaps between soda-lime glass and high-alumina glass in indicators such as hardness, impact resistance, and light transmittance directly determine a product’s user experience (e.g., whether it is easy to break or whether the screen display is clear) and service life.
Mechanical properties are the core of a cover glass’s "durability," with key indicators being hardness and impact resistance:
- Hardness: The industry commonly uses "pencil hardness" for measurement (higher hardness means stronger scratch resistance). After chemical strengthening, high-alumina glass can reach a hardness of 8H, and scratches from daily items such as keys and coins will not leave marks. Even after strengthening, soda-lime glass only has a hardness of 6H-7H, and fine scratches are likely to appear after long-term use (e.g., wear on a smartphone screen without a protective film).
- Impact resistance: According to the "ball drop test" standard (simulating object drop impact), with the same thickness (0.7mm-1.1mm), the drop resistance height of high-alumina glass can reach 1.5 meters—more than 1.8 times that of soda-lime glass (≤0.8 meters). This is due to the higher elastic modulus brought by high alumina content (approximately 72 GPa for high-alumina glass and 68 GPa for soda-lime glass). A higher elastic modulus allows the glass to "buffer and disperse stress" more effectively when impacted, reducing the risk of breakage (similar to how a spring is less likely to break when under force).
- Optical properties: Light transmittance determines the glass’s ability to transmit light and directly affects display quality. High-alumina glass generally has a light transmittance of over 91% without obvious color cast, enabling accurate reproduction of screen colors (e.g., the "True Tone display" of high-end smartphones relies on this property). Due to small amounts of iron impurities in its raw materials, soda-lime glass has a light transmittance of only 88%-90% and tends to show a slight bluish-green tint (visible when observing the edge of ordinary glass). It is more suitable for scenarios with no display requirements, such as windows and bottles.
- Thermal properties: The coefficient of thermal expansion measures the degree of expansion of a substance when heated. Soda-lime glass has a high coefficient of thermal expansion (approximately 95×10⁻⁷/°C) and is prone to cracking under drastic temperature changes (e.g., a glass bowl that has just held boiling water suddenly coming into contact with cold water). High-alumina glass has a low coefficient of thermal expansion; in an environment of -40°C to 85°C (a common temperature range for in-vehicle and outdoor equipment), its dimensional change rate is only 70% of that of soda-lime glass. This prevents the glass from "peeling off" from the screen module due to temperature fluctuations (e.g., in-vehicle screens will not warp after being exposed to intense sunlight in summer).

Cover glass substrates can be further divided into float glass and overflow fusion glass based on production processes. Currently, all soda-lime glass is produced using the float process, resulting in three types of cover glass substrates on the market: float soda-lime glass cover substrates, float high-alumina glass cover substrates, and overflow high-alumina glass cover substrates.
The float process has inherent defects in manufacturing high-alumina glass (uneven density causes warping after strengthening), leading to lower product quality compared to the overflow fusion process and low processing yield for midstream cover glass manufacturers. Therefore, in terms of performance, overflow high-alumina glass is the best, and it currently dominates the high-end market.
The conventional production method for glass is the float process:
Molten glass flows continuously from a melting furnace and floats on the surface of molten tin (which has a higher density). Under the action of gravity and surface tension, the molten glass spreads out and flattens on the molten tin surface, forming a glass ribbon with flat upper and lower surfaces. After hardening and cooling, the ribbon is pulled onto a transition roller table. The rollers of the table rotate to pull the glass ribbon out of the tin bath and into an annealing lehr. After annealing and cutting, flat glass products are obtained.
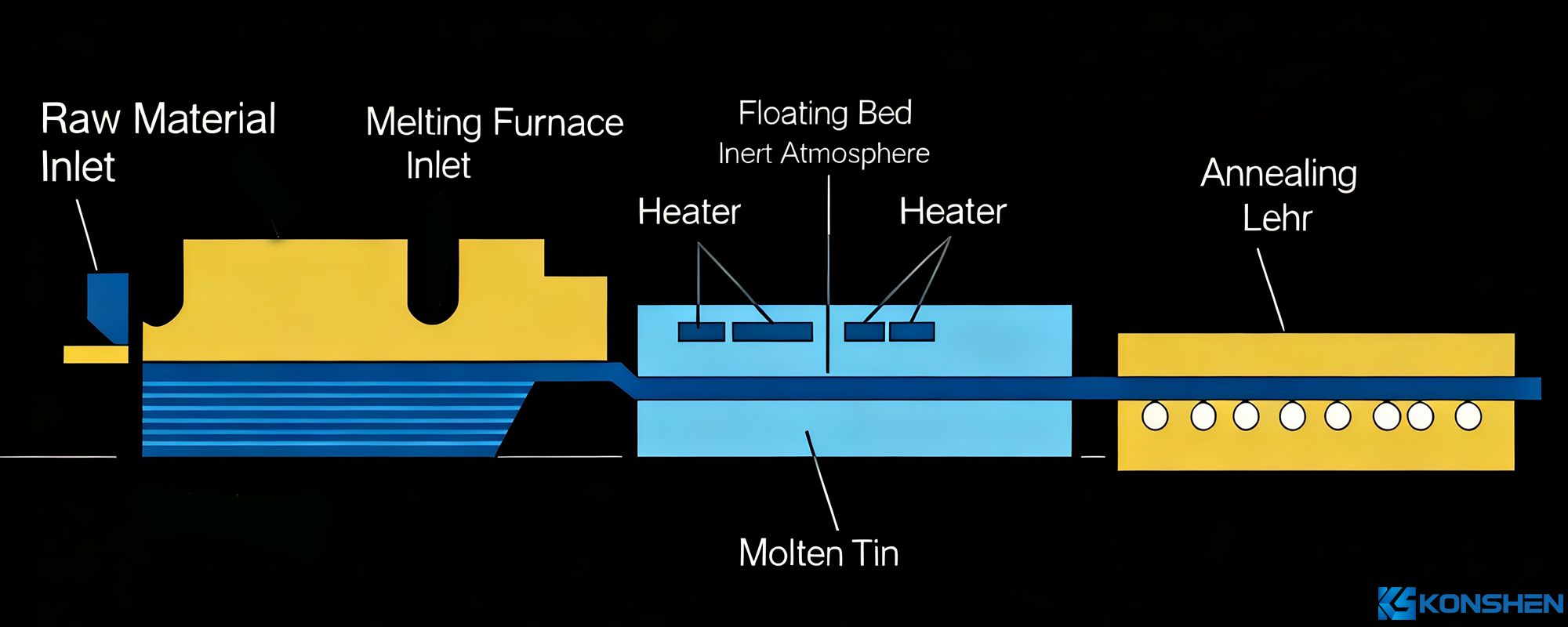
Soda-lime glass, medium-alumina glass, and high-alumina glass all fall under the category of float glass.
The overflow fusion process is an advanced technology for manufacturing high-quality flat glass, widely used in fields such as liquid crystal displays (LCDs) and organic light-emitting diode (OLED) displays. This process can produce glass substrates with extremely high flatness and optical quality.
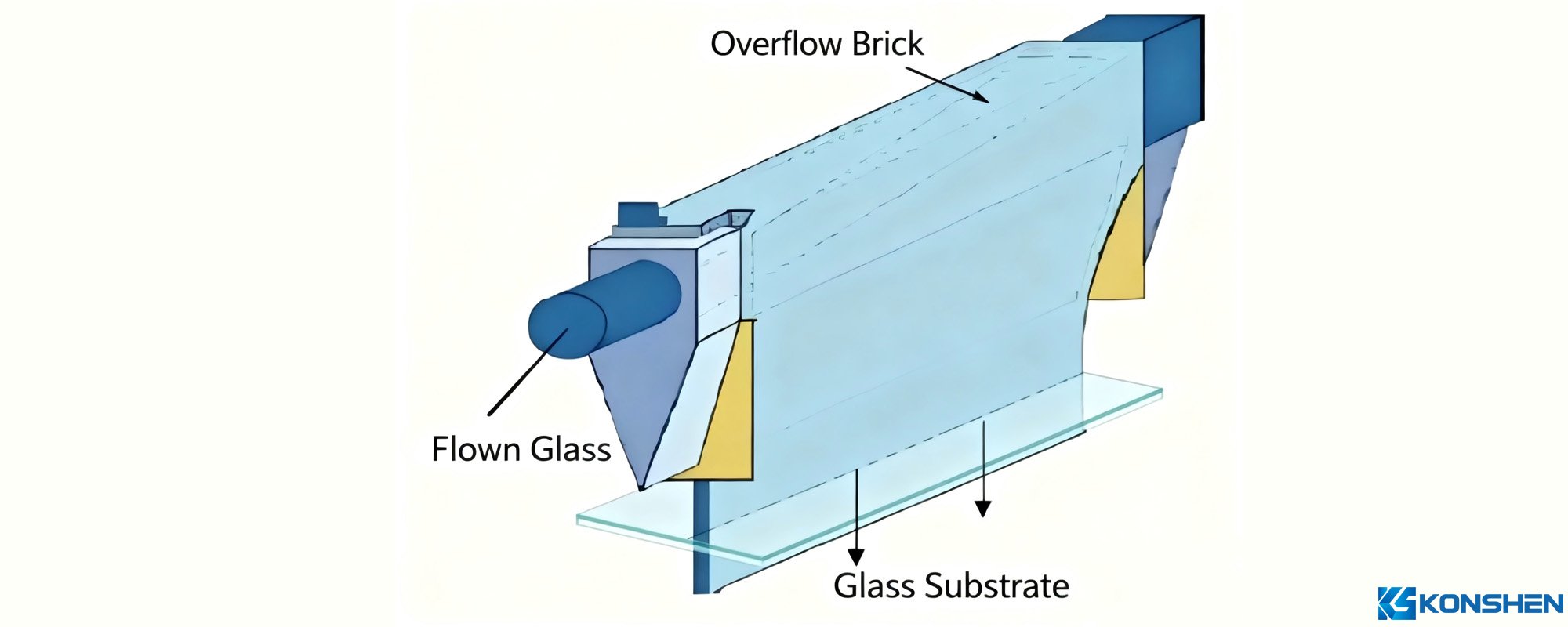
- Molten glass is poured into an overflow mold, which has a narrow notch at the top. The glass flows out of the notch and runs down both sides of the mold.
- Under gravity, the glass flowing down both sides converges at the bottom to form a flat glass ribbon. The thickness and quality of the glass can be adjusted by controlling the overflow rate and mold temperature.
- The overflow fusion process can produce ultra-high-quality flat glass that is ripple-free, bubble-free, and inclusion-free.
Both types of glass need to be strengthened through an ion exchange process (similar to "putting on a protective coat" for the glass): the glass is immersed in a high-temperature molten potassium salt bath, where the small-radius sodium ions (Na⁺) on the glass surface are replaced by large-radius potassium ions (K⁺). This forms a "compressive stress layer," improving the glass’s shatter and scratch resistance. However, there are significant differences in the process requirements for the two types:
- High-alumina glass requires a longer strengthening time (4-6 hours, compared to only 2-3 hours for soda-lime glass) and precise control of temperature (420°C-450°C) and salt bath concentration. The final "Depth of Layer (DOL)" of the stress layer can reach over 12 μm (a deeper stress layer means stronger shatter resistance).
- The soda-lime glass process is simple and has low requirements for equipment precision, making it suitable for large-scale production (e.g., ordinary home appliance panels). However, its stress layer is only about 8 μm thick, with poor stress uniformity, and "stress concentration" is likely to occur at the edges (e.g., the corners of the glass are more prone to breaking when dropped).
- Ultra-thin forming: Products such as foldable smartphones and ultra-thin laptops require ultra-thin glass (≤0.5mm). Due to its uniform composition, high-alumina glass can stably produce such products, with a flatness error of ≤0.1mm/m (equivalent to a height difference of no more than 0.1mm for a 1-meter-long glass). Soda-lime glass has large composition fluctuations, making it prone to "warping" (similar to the curvature of a potato chip) during ultra-thin production; it can usually only produce products with a thickness of ≥0.7mm.
- Surface treatment: High-end products often require coating the glass surface with Anti-Glare (AG), Anti-Reflection (AR), or Anti-Fingerprint (AF) coatings (e.g., the "anti-fingerprint film" on smartphone screens). High-alumina glass has high surface activity, and the coating adhesion can reach Grade 5B (no coating peeling in the cross-cut test). Soda-lime glass has weak surface adhesion, so its coating is prone to wear and peeling, requiring additional pretreatment (e.g., sandblasting), which instead increases costs.

The core logic for selecting cover glass is "scenario matching"—there is no need to blindly pursue high-alumina glass, nor rely solely on soda-lime glass to control costs. A comprehensive judgment should be made based on product positioning, usage environment, and budget.
- Consumer electronics: Entry-level smartphones, affordable tablets, and children's learning tablets. Users have low requirements for "durability" for these products, and cost control is a priority.
- Smart home: Basic light switches, display panels for rice cookers/washing machines. The usage environment is mild, with no frequent impact or scratching (e.g., switches are only pressed daily, with no drop risk).
- Industrial equipment: Indoor cash registers, warehouse barcode scanners. These are mostly used indoors, with low requirements for display quality (e.g., barcode scanning clarity) and temperature stability.

- Consumer electronics: Mid-to-high-end smartphones, flagship tablets, and laptops. Users value "screen texture" and "shatter resistance" and are willing to pay a premium for performance.
- In-vehicle field: In-vehicle central control screens, instrument panel covers. These need to withstand high temperatures from summer sun exposure (>60°C), low temperatures in winter (<-20°C), and driving vibrations, while also enduring frequent touches.
- Smart home: High-end smart switches (with touchscreens), display areas of smart door locks. These enhance texture and require wear resistance for long-term use (e.g., door lock panels are frequently touched by fingers).
- Industrial equipment: Outdoor charging piles, ATM screens, medical equipment panels. These need to resist outdoor impacts (e.g., hailstones hitting charging piles) and chemical corrosion (e.g., alcohol disinfection for medical equipment).
|
Selection Criterion |
Soda-Lime Glass |
High-Alumina Silicate Glass |
|
Core Composition |
SiO₂ (70%-75%), Na₂O, CaO; Al₂O₃ <13% |
SiO₂ matrix; Al₂O₃ (13%-24%); MgO/K₂O |
|
Key Performance |
- Hardness: 6H-7H (strengthened) |
- Hardness: 8H (strengthened) |
|
Processing |
- Chem. Strengthening: 2-3h, DOL≈8μm |
- Chem. Strengthening: 4-6h, DOL≥12μm |
|
Cost |
Low (1/3-1/2 of high-alumina) |
High (raw material + process costs) |
|
Applicable Scenarios |
1. Mid-low-end electronics |
1. Mid-high-end electronics |
|
Core Selection Fit |
Cost priority; mild environments; low durability/display demands |
High durability/visual effects; resists complex environments (high temp/impact/corrosion) |
Soda-lime glass and high-alumina glass are not "opposed as superior or inferior" but "each has its own strengths": the former supports mass-market demand with low costs, while the latter empowers high-end products with high performance. In actual selection, two extremes should be avoided: neither blindly assuming "high-alumina glass = high quality" (e.g., using high-alumina glass for children's learning tablets adds unnecessary costs) nor simply "reducing costs with soda-lime glass" (e.g., using soda-lime glass for in-vehicle screens makes them prone to cracking due to high temperatures).
Ready to take your glass project to the next level? Contact us today to discuss your custom glass needs and get a quote!
contact us